Molecular Weight Of Nitrogen Molecule: The Ultimate Guide For Science Enthusiasts
Ever wondered what the molecular weight of nitrogen molecule is and why it's so important? Well, buckle up because we're diving deep into this fascinating topic. Whether you're a student, a science geek, or just someone curious about the building blocks of our universe, you're in for a treat. In this article, we'll break down everything you need to know about the molecular weight of nitrogen molecules, and trust me, it's more interesting than you think.
Let's face it, science can be intimidating at times, but it doesn't have to be. By the end of this guide, you'll not only understand the concept of molecular weight but also how it applies to nitrogen molecules. We'll explore its significance in chemistry, biology, and even everyday life. So, whether you're brushing up on your knowledge or learning something new, you're in the right place.
Before we jump into the nitty-gritty details, let's set the stage. Nitrogen is one of the most abundant elements on Earth, making up about 78% of the air we breathe. It plays a critical role in countless biological processes, from plant growth to human DNA. Understanding its molecular weight helps us unlock secrets about how nature works. Ready to get started? Let's go!
- Ronnie Mcnutt The Life Legacy And Impact Of A Forgotten Icon
- How Old Is Peeta From The Hunger Games A Deep Dive Into The Boy With The Bread
What is Molecular Weight Anyway?
Alright, first things first. Molecular weight, also known as molar mass, is basically the weight of a molecule measured in grams per mole (g/mol). Think of it like weighing an entire molecule on a super-precise scale. For nitrogen molecules (N2), this value tells us how much nitrogen weighs when it's in its natural state.
Here's the deal: every element on the periodic table has a specific atomic weight, and when atoms bond together to form molecules, their weights add up. For nitrogen, since it exists as N2 in nature, we simply double the atomic weight of nitrogen (14.0067 g/mol) to get the molecular weight. Easy, right?
But why does this matter? Well, molecular weight is crucial in chemistry because it helps us calculate how much of a substance we need for experiments, industrial processes, or even medical applications. It's like having a universal language for measuring chemicals.
- Chinese Horoscope Sign 1985 A Deep Dive Into The Year Of The Ox
- Chinese Star Sign 1974 Discover Your Zodiac Power And Destiny
Breaking Down the Nitrogen Molecule
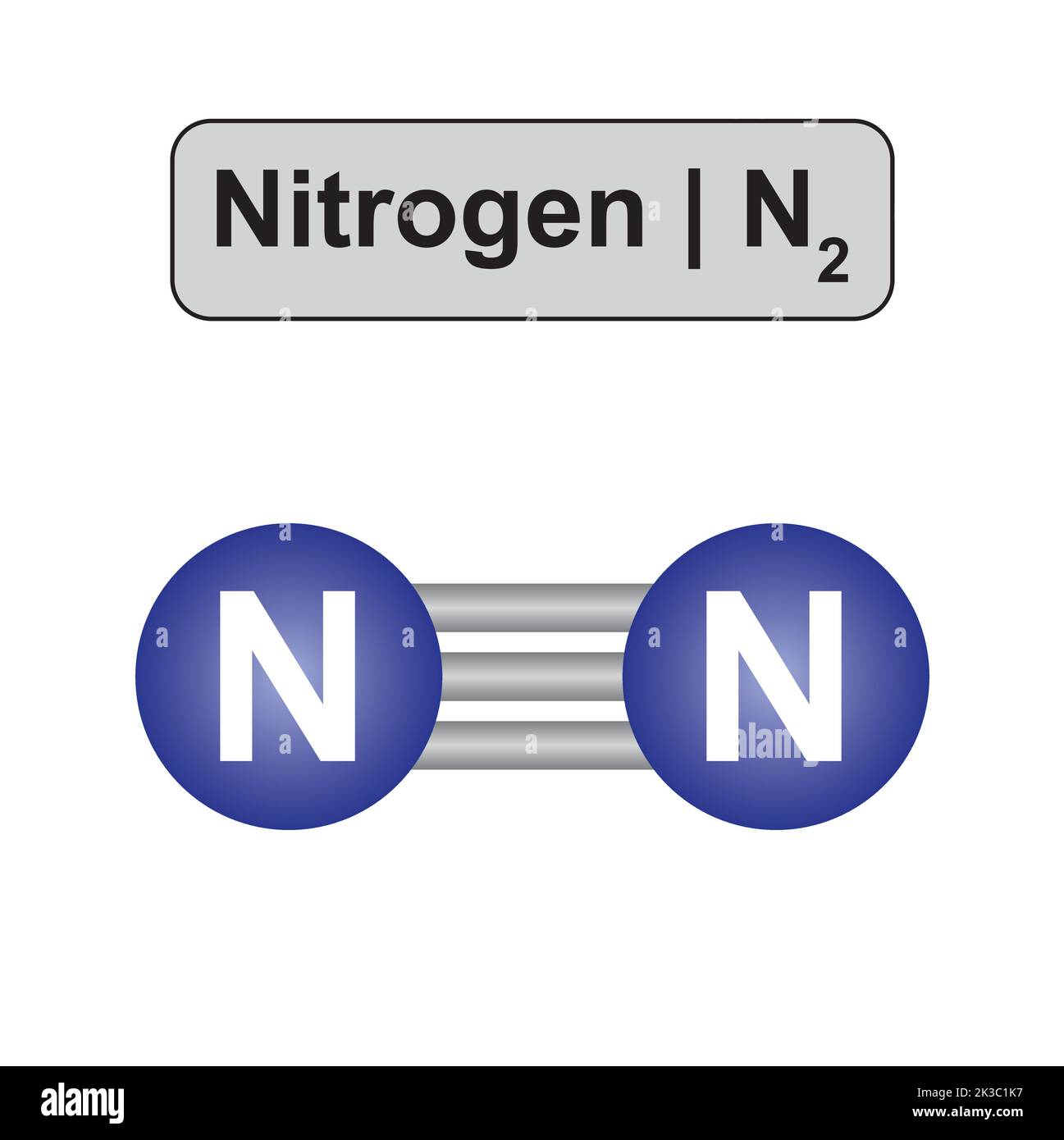
Now that we know what molecular weight is, let's zoom in on the nitrogen molecule itself. Nitrogen (N2) is made up of two nitrogen atoms bonded together. These atoms share electrons in a triple bond, making N2 one of the most stable molecules out there. Stability is key because it means nitrogen doesn't react easily with other substances under normal conditions.
Why Is Nitrogen So Stable?
The secret lies in that triple bond. It's super strong, requiring a lot of energy to break apart. That's why nitrogen gas (N2) is mostly inert and doesn't participate in chemical reactions unless you really push it, like in high-temperature environments or with catalysts.
- Nitrogen atoms are held together by three covalent bonds.
- This triple bond makes N2 highly unreactive under normal conditions.
- Breaking the bond requires a lot of energy, which is why nitrogen is stable.
The Molecular Weight of Nitrogen Molecule
So, what exactly is the molecular weight of nitrogen molecule? Drumroll, please! The molecular weight of N2 is approximately 28.0134 g/mol. This number comes from adding up the atomic weights of the two nitrogen atoms in the molecule.
Here's the math for those who love numbers:
Atomic weight of nitrogen (N) = 14.0067 g/mol
Molecular weight of N2 = 14.0067 + 14.0067 = 28.0134 g/mol
Simple, right? But don't let the simplicity fool you. This number has far-reaching implications in science and industry.
Applications of Nitrogen's Molecular Weight
Knowing the molecular weight of nitrogen isn't just for academic purposes. It has real-world applications that affect everything from agriculture to space exploration. Here are a few examples:
1. Fertilizer Production
Nitrogen is a key ingredient in fertilizers, which help plants grow strong and healthy. By understanding the molecular weight of nitrogen, scientists can calculate exactly how much nitrogen to add to soil for optimal plant growth.
2. Industrial Uses
In industries like electronics and pharmaceuticals, nitrogen is used to create inert atmospheres. Its molecular weight helps engineers determine how much nitrogen gas is needed for specific processes.
3. Environmental Science
Nitrogen plays a critical role in the nitrogen cycle, a natural process that moves nitrogen through the atmosphere, soil, and living organisms. Understanding its molecular weight helps scientists study and mitigate issues like nitrogen pollution in waterways.
Understanding the Importance of Molecular Weight
Molecular weight isn't just a number—it's a powerful tool. It allows scientists to predict how molecules will behave in different conditions, calculate reaction rates, and design new materials. For nitrogen, this knowledge is especially important because of its widespread use in various fields.
Think about it: without knowing the molecular weight of nitrogen, we wouldn't be able to produce the fertilizers that feed billions of people or create the inert atmospheres needed for advanced manufacturing. It's like having a secret code to unlock the potential of one of the most abundant elements on Earth.
How Molecular Weight Affects Nitrogen's Properties
The molecular weight of nitrogen influences many of its physical and chemical properties. For example, its relatively low molecular weight makes nitrogen gas less dense than air, which is why it rises in the atmosphere. This property is crucial for maintaining the balance of gases in our environment.
Additionally, nitrogen's molecular weight affects its boiling and melting points. Nitrogen gas becomes a liquid at -195.8°C (-320.4°F), a property that's exploited in cryogenics and other low-temperature applications.
Common Misconceptions About Molecular Weight
There are a few myths floating around about molecular weight that we need to clear up. For instance, some people think that heavier molecules are always more reactive. Wrong! Stability depends on factors like bond strength, not just weight. Nitrogen is a great example of this—despite its relatively low molecular weight, it's incredibly stable.
Myth vs. Reality
- Myth: Heavier molecules are always more reactive.
- Reality: Reactivity depends on bond strength and molecular structure.
- Myth: Molecular weight is only important in chemistry.
- Reality: It has applications in biology, physics, and engineering too.
Historical Context of Nitrogen Discovery
Nitrogen was first discovered in 1772 by a Scottish physician named Daniel Rutherford. He isolated nitrogen by removing oxygen and carbon dioxide from air, leaving behind a gas that didn't support combustion or life. Back then, they called it "noxious air" because it seemed harmful. Little did they know, nitrogen would turn out to be one of the most important elements in nature.
Since then, scientists have learned a lot about nitrogen, including its molecular weight and properties. This knowledge has paved the way for countless innovations in science and technology.
Conclusion: Why Knowing the Molecular Weight of Nitrogen Matters
There you have it—the molecular weight of nitrogen molecule is approximately 28.0134 g/mol. But it's so much more than just a number. It's a key to understanding one of the most fundamental elements in our universe. From feeding the world to exploring space, nitrogen plays a vital role in our lives, and knowing its molecular weight helps us harness its potential.
So, what's next? If you found this article helpful, feel free to share it with your friends or leave a comment below. And if you're hungry for more science knowledge, check out our other articles on chemistry, biology, and physics. The world of science is vast and exciting, and there's always something new to discover. Stay curious, my friend!
Table of Contents
- What is Molecular Weight Anyway?
- Breaking Down the Nitrogen Molecule
- The Molecular Weight of Nitrogen Molecule
- Applications of Nitrogen's Molecular Weight
- Understanding the Importance of Molecular Weight
- How Molecular Weight Affects Nitrogen's Properties
- Common Misconceptions About Molecular Weight
- Historical Context of Nitrogen Discovery
- Conclusion: Why Knowing the Molecular Weight of Nitrogen Matters
- Exploring Marcy Projects Brooklyn A Deep Dive Into Nycs Iconic Housing Community
- Ben Shapiro Nationality The Story Behind The Popular Commentators Roots
Nitrogen molecule icon consisting of Nitrogen. Flat. Vector
Molecular Model of Nitrogen (N2) Molecule. Vector Illustration Stock

Nitrogen Molecule Structure